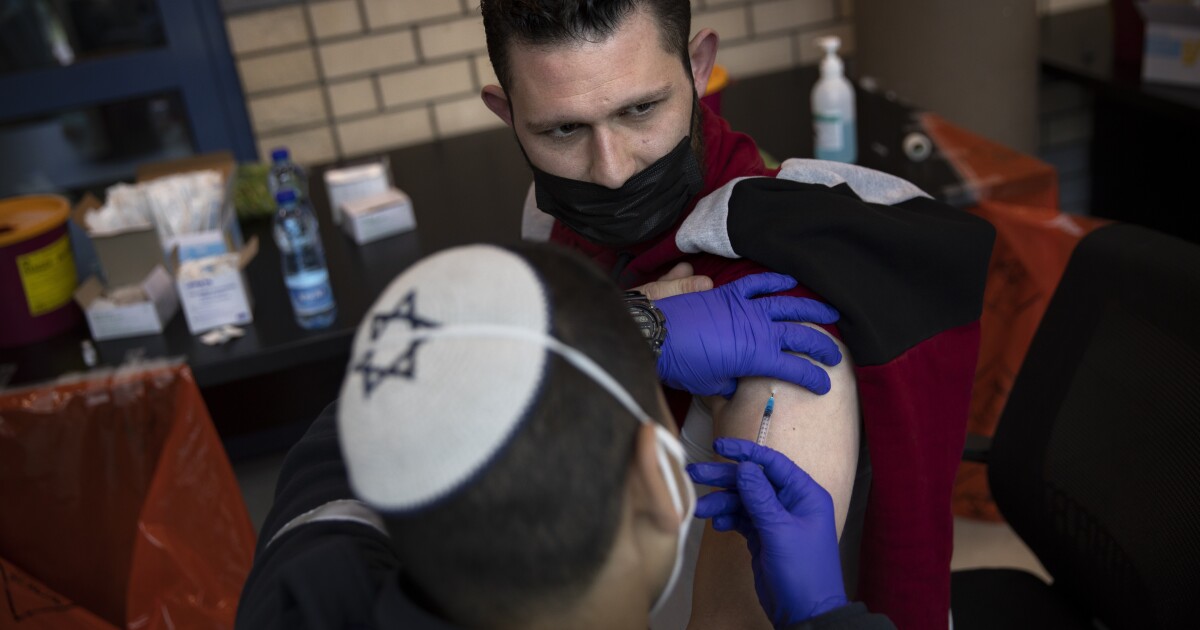
In clinical trials conducted last fall, the vaccine developed by Pfizer and BioNTech proved 95% effective in preventing symptoms of COVID-19.
The question remained whether it and other vaccines would perform as well once they went into widespread use.
A new study involving more than half a million people who were vaccinated in Israel strongly suggests the answer is yes.
Compared with people who did not receive the Pfizer-BioNTech vaccine, those who were inoculated were 94% less likely to become ill, according to the study published Wednesday in the New England Journal of Medicine. They also had far lower rates of death, hospitalization and — among those who were tested for the virus — infection.
Josh Michaud, a global health expert at the Kaiser Family Foundation who wasn’t involved in the study, called it an “important milestone.”
“Up until now we’ve had to rely on scattered small-scale reports and non-peer-reviewed findings for a glimpse of how effective COVID-19 vaccines are in the real world,” he said.
The research did not address the critical question of whether vaccination prevents a person from transmitting the virus.
Clinical trials of various vaccines have focused on whether participants developed symptoms, leaving open the possibility that even those protected from illness could still become infected and unknowingly infect others.
The new study did not systematically test people for the virus, so there was no way of measuring how many people without symptoms may have been infected.
However, among those who were tested — either because they got sick or they wanted to ensure that they were not infected — those who had received both doses of the vaccine were 92% less likely to get a positive result than members of a control group who had not been vaccinated.
Dr. Noam Barda, one of the authors, said research is now underway to rigorously test people who have been vaccinated to definitively determine whether vaccination prevents infection and transmission.
For now, Dr. Carlos del Rio, an infectious-disease researcher at Emory University who was not involved in the study, said seeing the high efficacy data carry over from clinical trials to the real world was “very encouraging.”
Though controlled clinical trials are the best way to test the effectiveness of a new vaccine, they are far from a perfect predictor of how a rollout will go — particularly one so urgent and fraught with challenges.
Vaccine recipients sometimes fail to adhere to the prescribed timelines for getting their shots. Vaccines must be shipped long distances and kept cold. Lots of things can go wrong and undermine effectiveness.
The new study used data on 596,618 people who received the Pfizer-BioNTech vaccine between Dec. 20 and Feb. 1. Another 596,618 who were not vaccinated were selected for a control group based on age, sex, neighborhood, preexisting conditions and other factors that could influence a person’s likelihood of contracting the virus or becoming ill.
All participants were members of Clalit Health Services, Israel’s largest healthcare organization.
Even after just one dose, the vaccinated group fared far better than their unvaccinated peers. They were 57% less likely to get sick and 74% less likely to be hospitalized. After the second and final dose, those figures rose to 94% and 87%.
Two to three weeks after the first shot, the vaccine was 72% effective at preventing death.
Jennifer Nuzzo, an epidemiologist at Johns Hopkins Bloomberg School of Public Health, said the findings “should give us good hope that we can use vaccines to prevent hospitalizations and death, which would effectively defang the virus.”
The vaccine worked similarly well across all age groups.
Nicholas Davies, an epidemiologist at the London School of Hygiene and Tropical Medicine who was not involved in the study, said a high degree of protection for the elderly — and only slightly lower protection for people with multiple chronic health problems — “gives reason to be optimistic about vaccine effectiveness in the most vulnerable populations.”
The study’s authors said they were also encouraged that the emergence of coronavirus variants did not seem to have a major impact on the effectiveness of the vaccine.
The clinical trials were conducted when most of the viruses in circulation were a close match to the one used to design the Pfizer-BioNTech vaccine. The new study did not look at which variants were most common among the participants, but B.1.1.7 — the so-called U.K. variant — made up a large share of overall cases in Israel by the end of the study period.
Israel is far ahead of most other countries in its vaccination campaign, with more than half the population inoculated.
https://ift.tt/3uxpGrp
Business

No comments:
Post a Comment